Breaking the Water Molecule: Electrolysis
2020-06-20
Use a simple homemade device to split a water molecule into two gases.
Questions to explore:
- What does a water molecule look like?
- How can we turn a liquid into a gas?
- What happens when two opposite charges get near each other?
What you'll need:
- One (1) 9V battery
- Two (2) stainless steel screws
- Rubber bands
- One (1) shallow dish (not metal)
- pH indicator (cabbage juice works great)
- Water
- Epsom salt*
- Spoon
*NOT table salt - see the How it Works section for reason
What to do:
- Assemble your electrolysis device: place the threads of each screw on each terminal of the battery (make sure the screws don’t touch!) Use rubber bands to hold them in place.
- Prepare your solution: fill the shallow dish with water and a pH indicator (approximately half-and-half). Mix in a spoonful of Epsom salt and stir until the salt is mostly dissolved.
- Place the two screws into the shallow dish (again, don’t let them touch). You should see something happen within seconds. Observe. What do you notice? Why is this happening?

How it works:
A water molecule (H2O) is made of two hydrogen atoms and an oxygen.
▲
Check out the minipost The Smallest Particles to learn more about atoms and molecules.
The hydrogen atoms have a positive charge and the oxygen atoms have a negative charge.
▲
See the minipost Polarity for a description of how this works.
It turns out that if you run electricity through water, you can break the liquid water molecule into its two gases, hydrogen (H) and oxygen (O).
We know that opposite charges attract.
▲
Check out the Balloon & Stream Demo for an exploration of opposite charges.
Since batteries have a positive and negative terminal,
▲
Look for a + on one side of your battery to know which is positive.
the positive hydrogen is drawn to the negative terminal and the negative oxygen is drawn to the positive terminal.
▲
The oxygen is actually still attached to one of the hydrogens in a negative compound called hydroxide
(OH-).
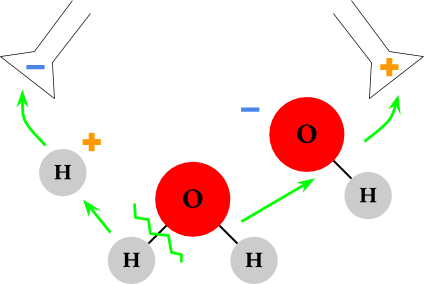
Let’s look at one side at a time to explain what’s happening. We’ll start with the negative battery terminal.
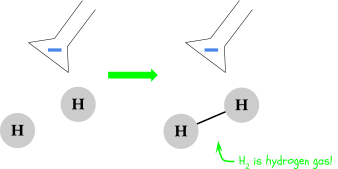
The hydrogen bonds together as H2, otherwise known as hydrogen gas. You’ll see small bubbles forming around the terminal - these are bubbles of hydrogen gas. Let’s see what’s happening at the positive terminal:
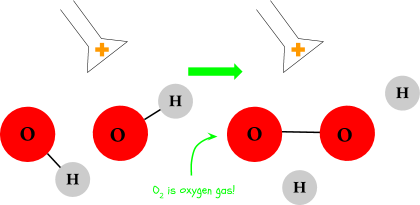
The oxygen in the hydroxide (OH-) breaks off and bonds together as O2, otherwise known as oxygen gas (this is the stuff we breathe). You’ll see some small bubbles forming around the terminal - these are bubbles of oxygen gas. There are roughly half as many oxygen bubbles as hydrogen bubbles; remember that water is one part oxygen and two parts hydrogen.
One last thing. Epsom salts, like all salts, conduct electricity really well. This helps the electricity from the battery move through the solution more quickly. The reason we can’t use table salt is because when you run an electric current through a salt, it breaks it up. Epsom salt splits into magnesium and sulfate, which are fairly harmless, but table salt (sodium chloride) would split into sodium and poisonous chlorine gas.