The Smallest Particles
2020-07-20
What is stuff made of?
The Atom
Meet the atom. The atom is the building block of all matter. In other words, everything around you is made up of atoms.
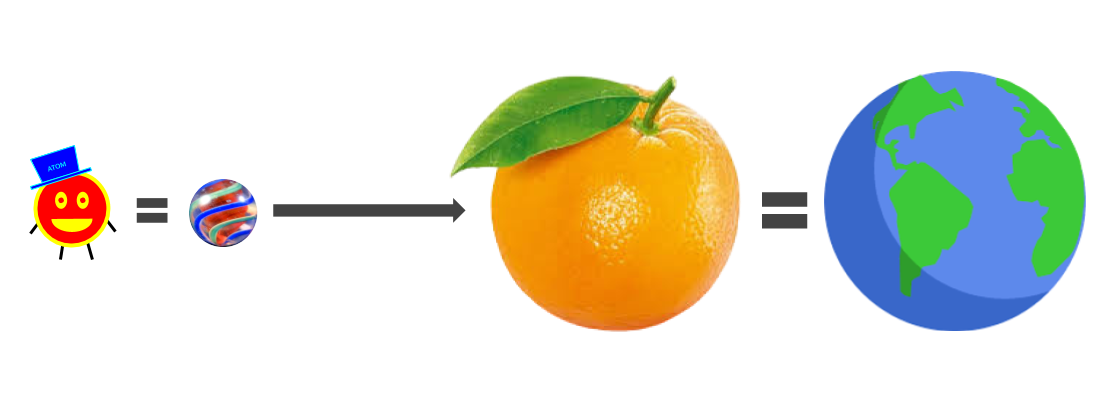
For having such a big responsibility, the atom is pretty small. How small? Well, let’s blow an atom up to the size of a marble. If an atom is blown up to the size of a marble, then an orange, which is made of trillions of atoms, would be about the size of the earth.
Atoms come in different types (see the elements section below). Combining different types of atoms in different ways gets you everything you see around you.
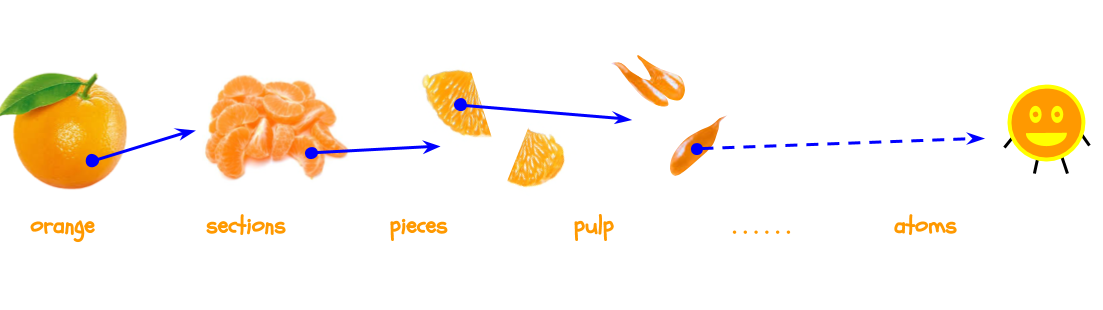
An atom is a fundamental particle. This means that it is the smallest unit of matter. Let’s go back to the orange. We know that an orange is made of sections. We can cut up a section, and now we have pieces that make up sections that make up the orange. If we cut up a piece, we’ll see that it’s made of pulp. Let’s pretend we have a magic knife that can keep cutting the orange into smaller and smaller pieces. Eventually, we’ll get to atoms, and we cannot cut atoms into anything smaller.
▲
The word atom comes from the ancient Greek atomos, literally meaning “indivisible”
Coming Together: Compounds and Molecules
Okay, so atoms are the smallest pieces of matter. But what comes right after atoms? What do you get when you combine atoms?
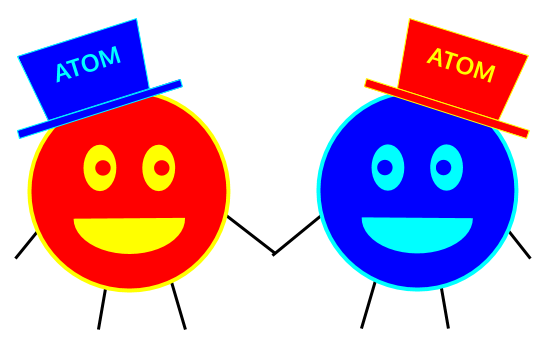
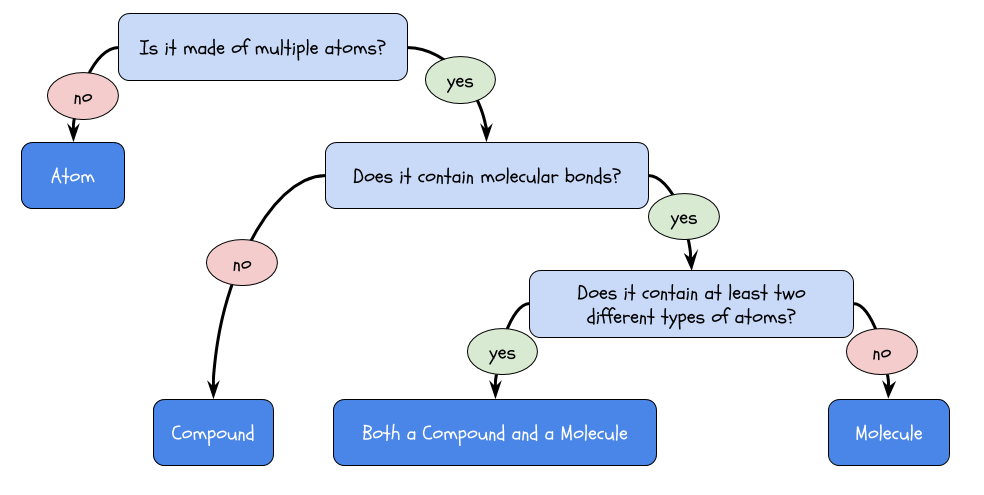
Compounds and molecules are made up of atoms. These are separate terms that both mean “clumps of multiple atoms with certain rules.” What are the rules? Well, for something to be a compound, it has to be made up of at least two types of atoms. Even if you have multiple oxygen atoms bonded together, it’s not an oxygen compound because that’s only one type of atom.
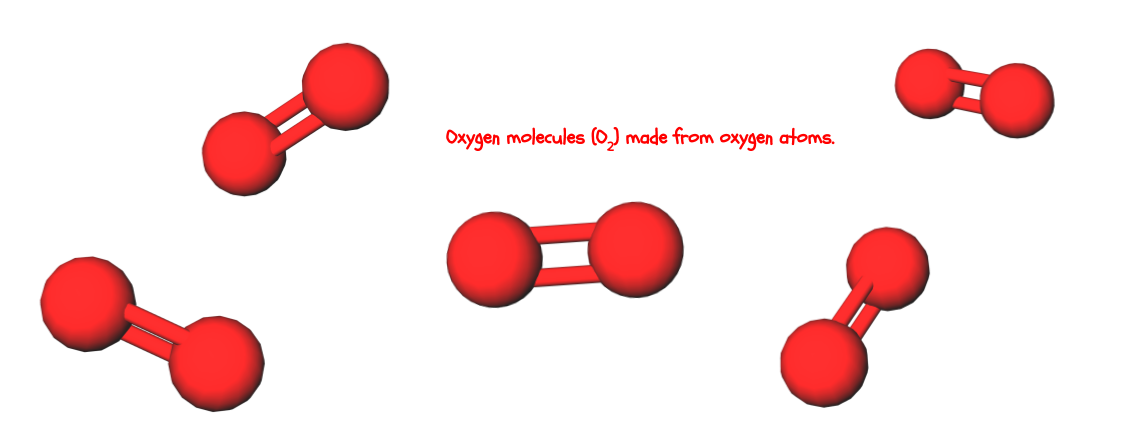
It is, however, an oxygen molecule. For something to be a molecule, the atoms have to be bonded in a certain way, called a molecular bond. You don’t need to worry about what a molecular bond is to know that a molecule requires this certain type of bond. Check out the last section for some examples of compounds and molecules.
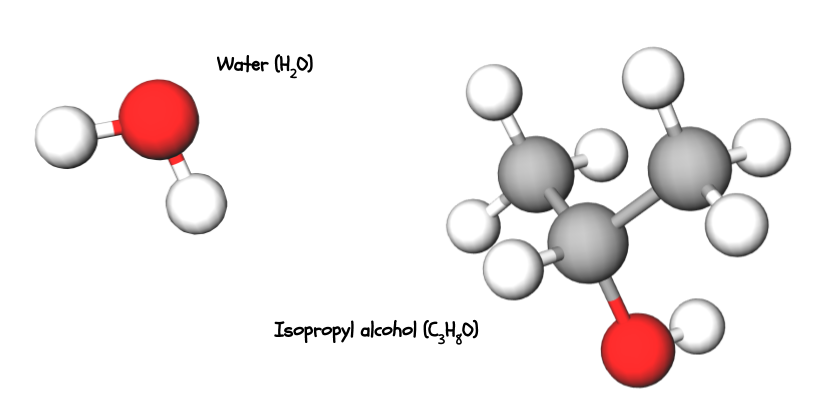
Compounds and molecules are written with chemical formulas. The little number (the subscript) after an atom abbreviation tells you how many of that type of atom there are. For example, water (H2O) has two hydrogen atoms and one oxygen atom. Isopropyl (rubbing) alcohol (C3H8O) has three carbon atoms, eight hydrogen atoms, and one oxygen atom.
Going Subatomic: Protons, Electrons, and Neutrons
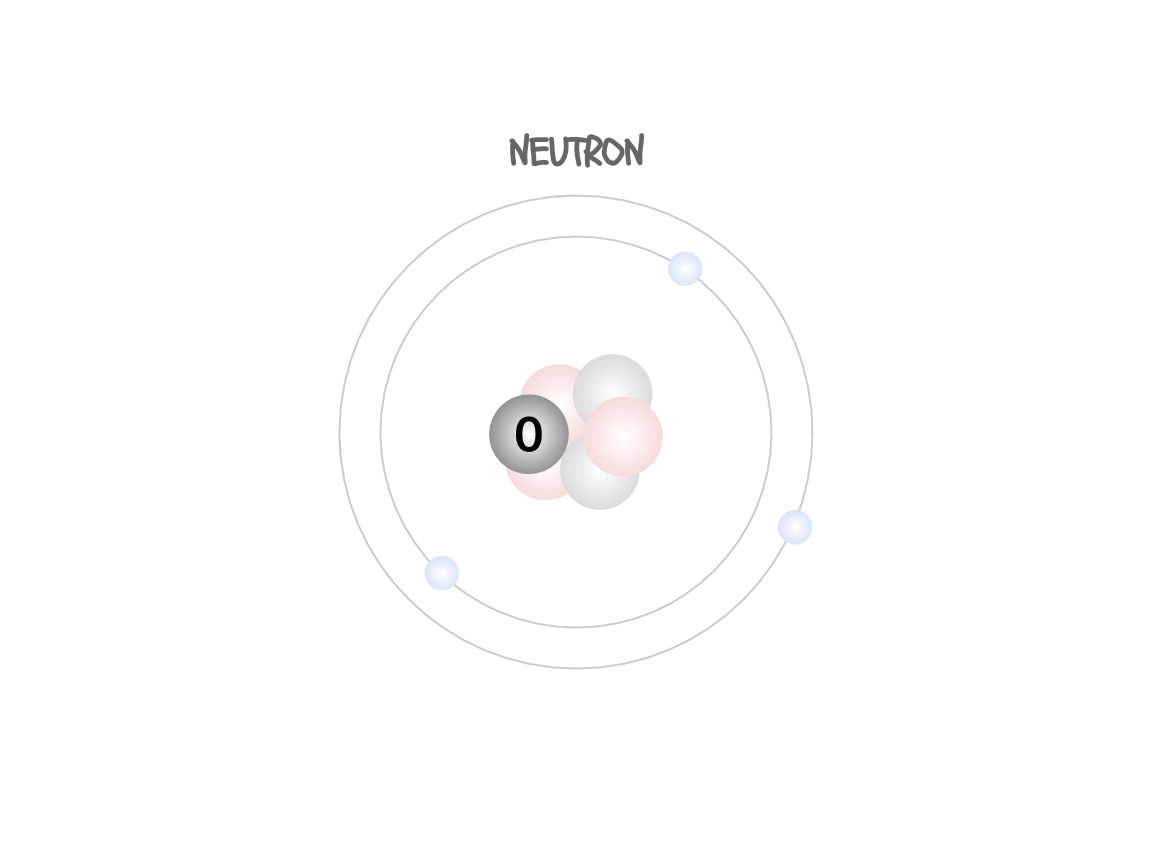
Even though atoms are considered the smallest unit of matter, they are made of some distinct parts (confusing, I know). These are called the subatomic particles. There are three of them: the proton, the electron, and the neutron.
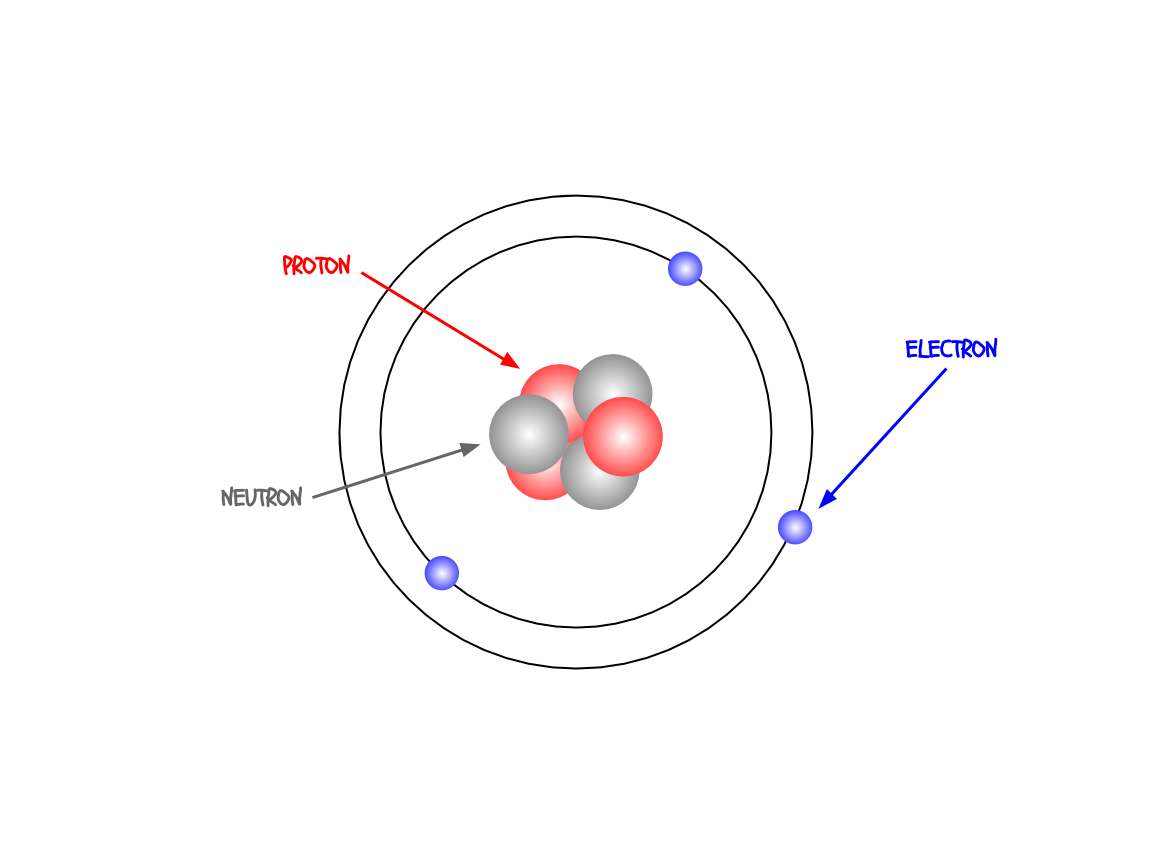
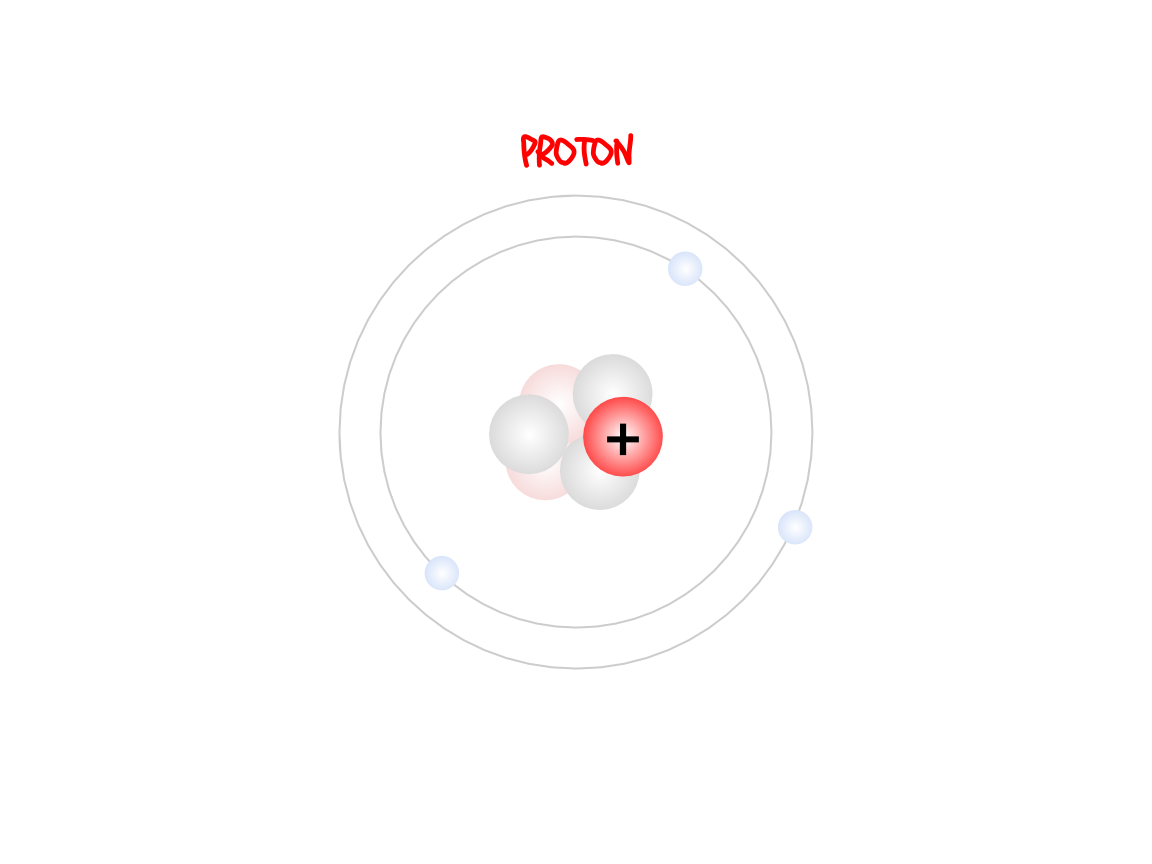
The protons are in the nucleus (the center) of the atom. They are positively charged and they tell you what type of atom you’re looking at.
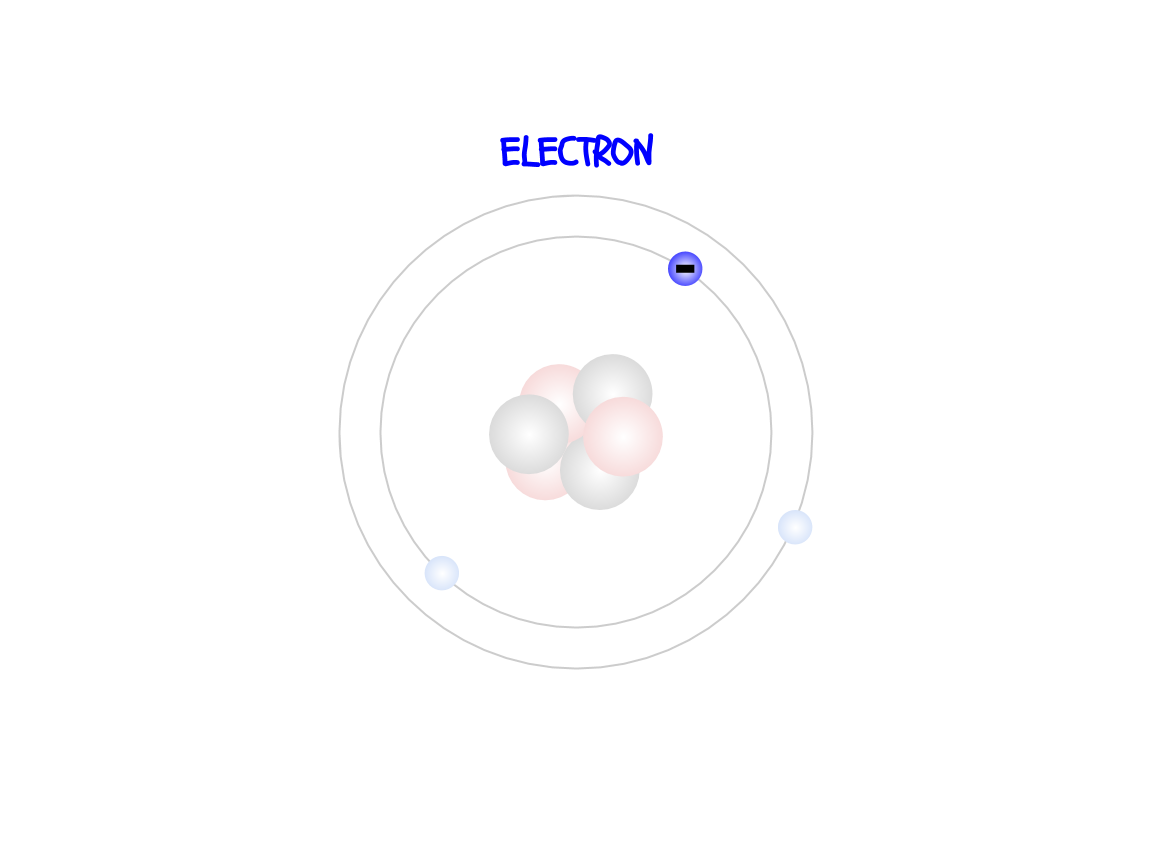
The electrons are in a ring
▲
Or more accurately, a cloud
around the nucleus. They are negatively charged and help the atom bond to other atoms and form compounds and molecules.
The neutrons are in the nucleus with the protons. They have no charge but they help stabilize the atom.
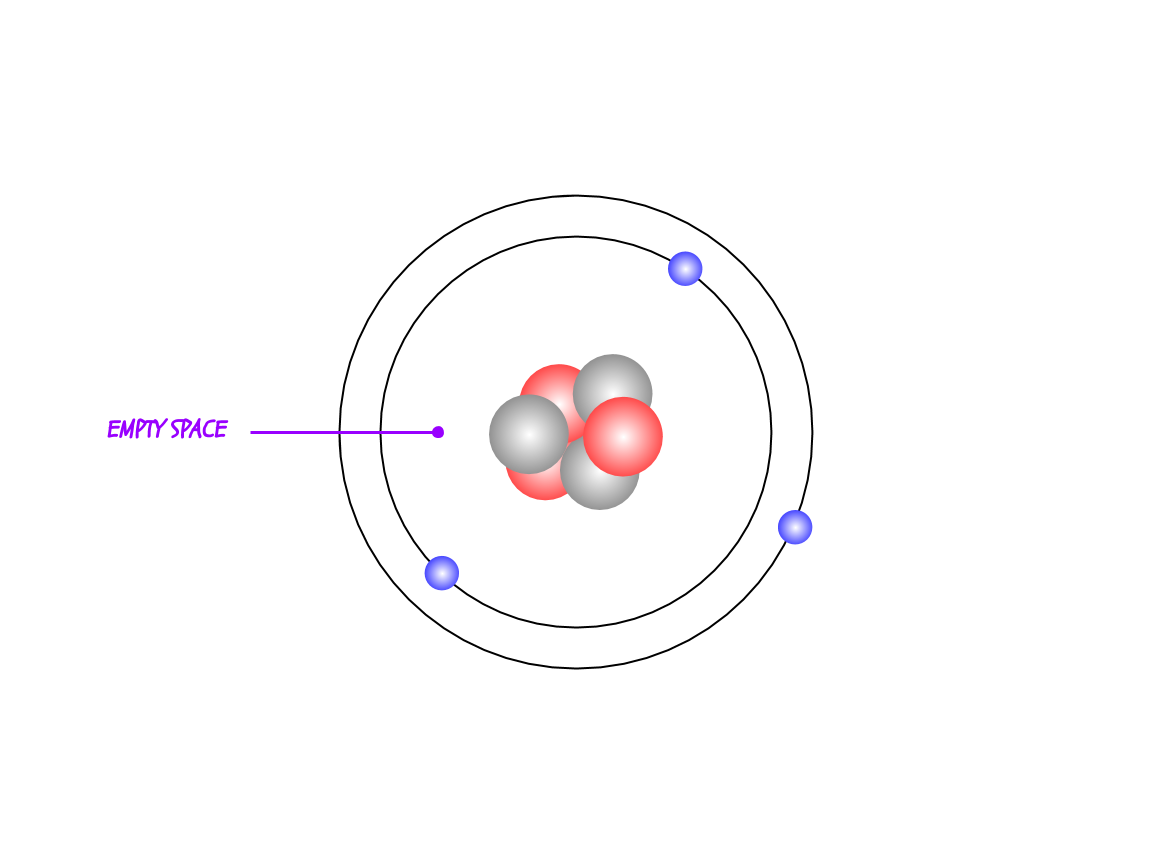
Atoms are mostly empty space. 99.9999999999996% empty space, to be exact. If you remove all the empty space in our atoms, then the entire human race would be the size of a sugar cube.
▲
Citation
Atom ID: The Elements
As mentioned above, you can tell what type of atom you are looking at by the number of protons. The holy grail of chemistry, the periodic table, lists the atoms in order by atomic number, which is equal to the number of protons. Look for C (carbon) on the table. Hint: it is a nonmetal, so the rectangle will be dark blue. See the little number 6 in the upper corner? That means carbon always has 6 protons. How many protons does an atom of aluminum (Al) have? How about molybdenum (Mo)? Mendelevium (Md)?
▲
Aluminum: 13
Molybdenum: 42
Mendelevium: 101
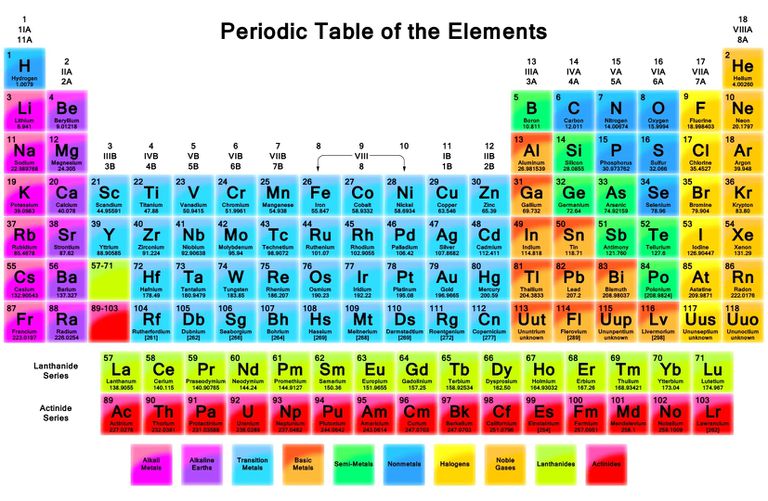
Image credit: thoughtco.com
Charging Up: Ions
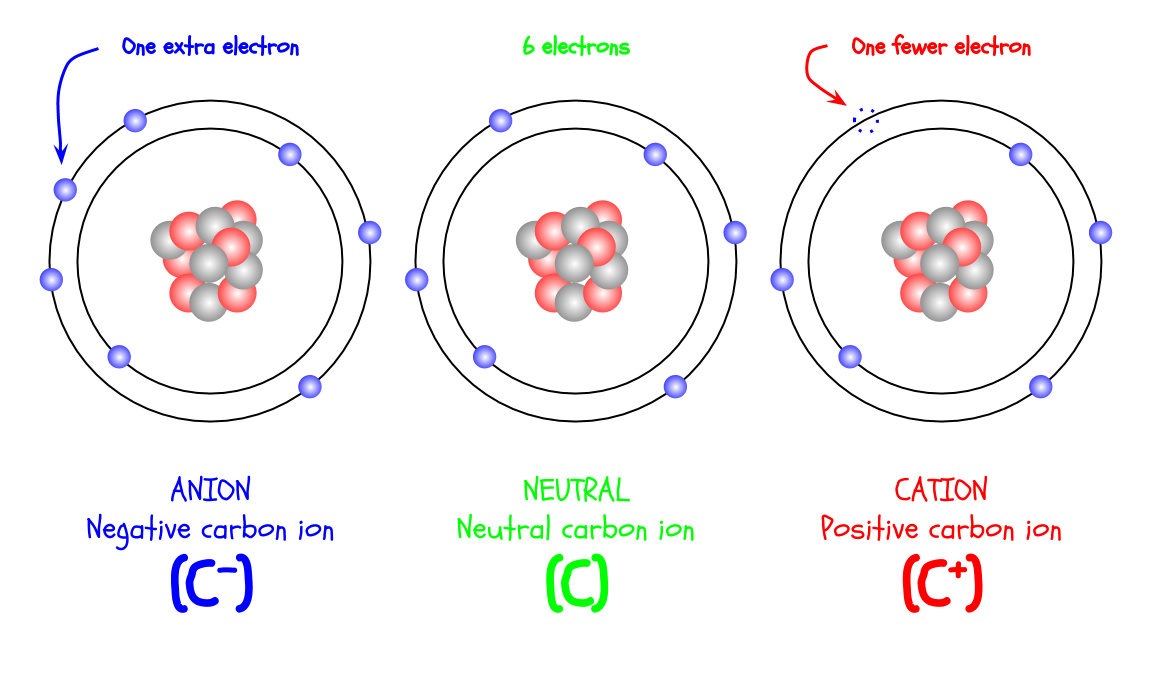
Even though carbon always has 6 protons, the number of electrons can change. Since electrons are negatively charged, changing the number of electrons changes the charge of the atom. If a carbon atom has 6 electrons, it is neutrally charged (charge of 0) because each electron charge cancels out a proton charge.
If you add an electron to a carbon atom, it has a charge of -1. If you take an electron, it has a charge of +1. Atoms with different numbers of electrons are called ions. Ions with negative charges are anions, while ions with positive charges are cations.
▲
A trick to remember this: CATions are “PAWS-itive”
An Example: Zooming In
Let’s take a glass of salt water. What are some of the particles we have?

Firstly, we have a bunch of water molecules in the glass. Water molecules are made of 3 atoms: two hydrogen atoms and an oxygen atom. Since there are two different types of atom in water, a water molecule is also a compound.

The air above the water also contains molecules. Mostly, it is oxygen molecules and nitrogen molecules. These are not compounds because they both contain two of the same type of atom.
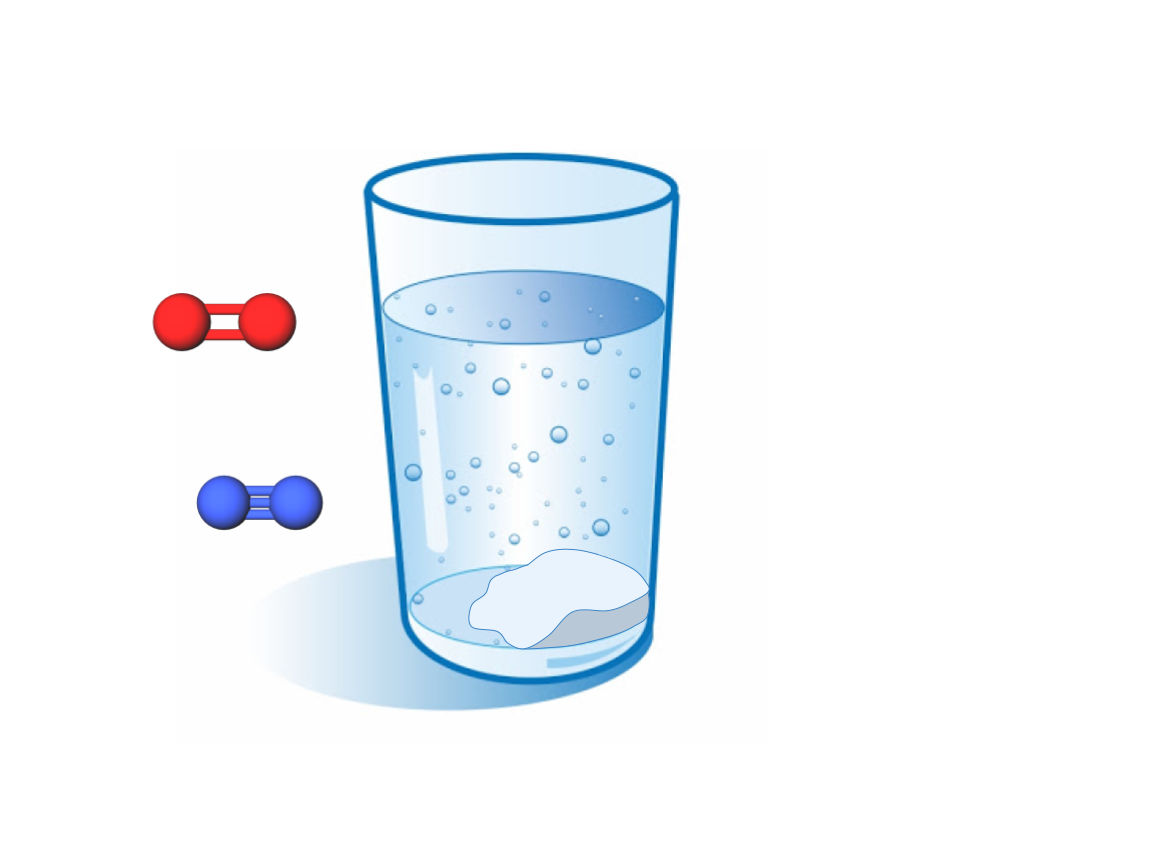
Some of the salt in the water is made of compounds. A salt compound has one sodium atom per one chlorine atom. There are no molecular bonds in salt, so salt is not a molecule.

Since salt dissolves in water, the two atoms split up into ions. When the atoms separate, the chlorine will “steal” an extra electron from the sodium and be negatively charged. The sodium, short one electron, will be positively charged.

The glass that holds the salt water is mostly silicon dioxide, which is both a molecule and a compound. Each glass molecule has one silicon atom and two oxygen atoms.

Why do we care about the smallest particles?
As you can see, every simple thing, like a glass of salt water, is made up of many different atoms, molecules, compounds, and ions. Anything we can touch in our world is matter, and all matter is made up of these smallest particles. If we want to understand how something works on a macroscopic level, it’s important to zoom in and see what’s going on on the microscopic level.
▲
Macroscopic = anything we can see with the naked eye. Microscopic = anything too small to see with the naked eye.
The workings of atoms make the entire world work the way it does.