Acids, Bases, and the pH Scale
2020-07-20
What makes something acidic or basic? How is acidity measured?
Acids and bases
Chemical substances can be classified as being an acid or a base.
Here are some of the physical differences between acids and bases:
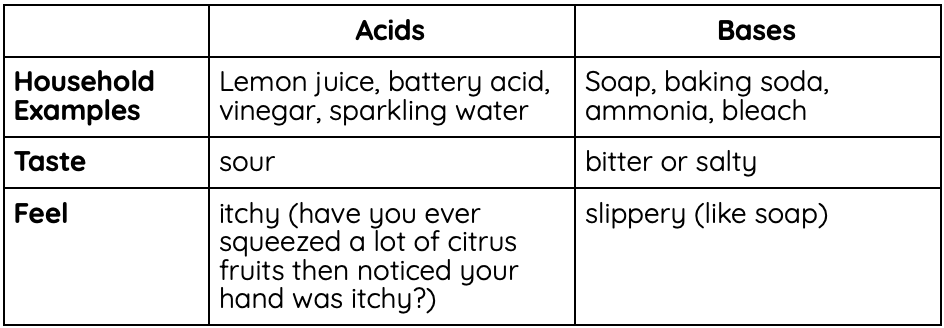
Even though acids have a scarier reputation, both acids and bases can be harmful to us. Both acids and bases can also be completely safe. We drink acidic lemonade and sparkling water all the time, and clean ourselves with basic soap. It’s very important to always take safety measures (gloves, goggles, etc.) when handling unknown acids and bases, but there’s no need to fear either one.
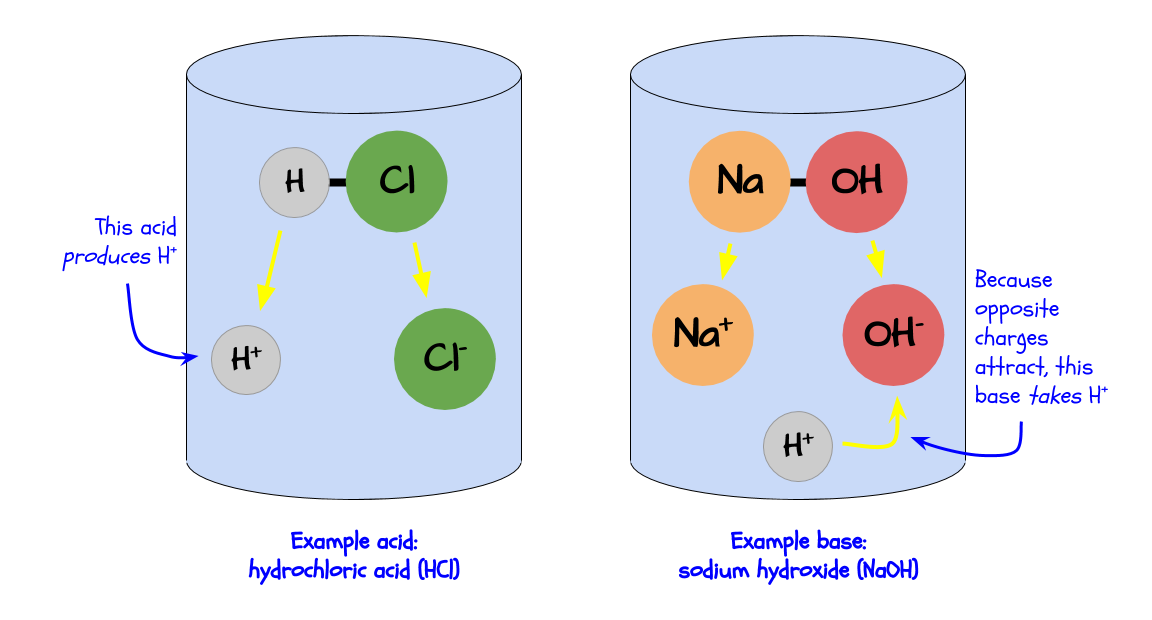
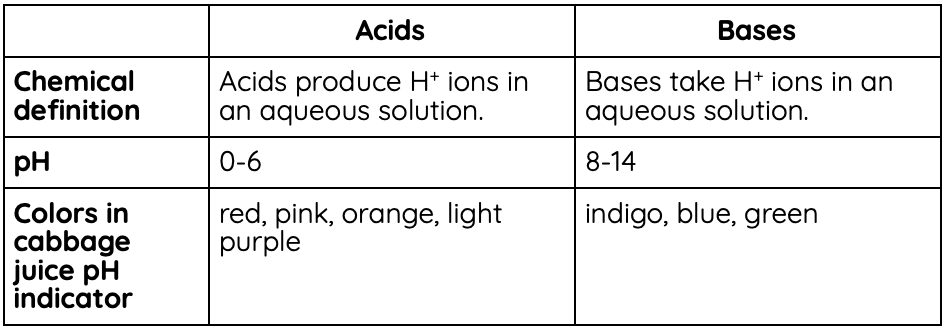
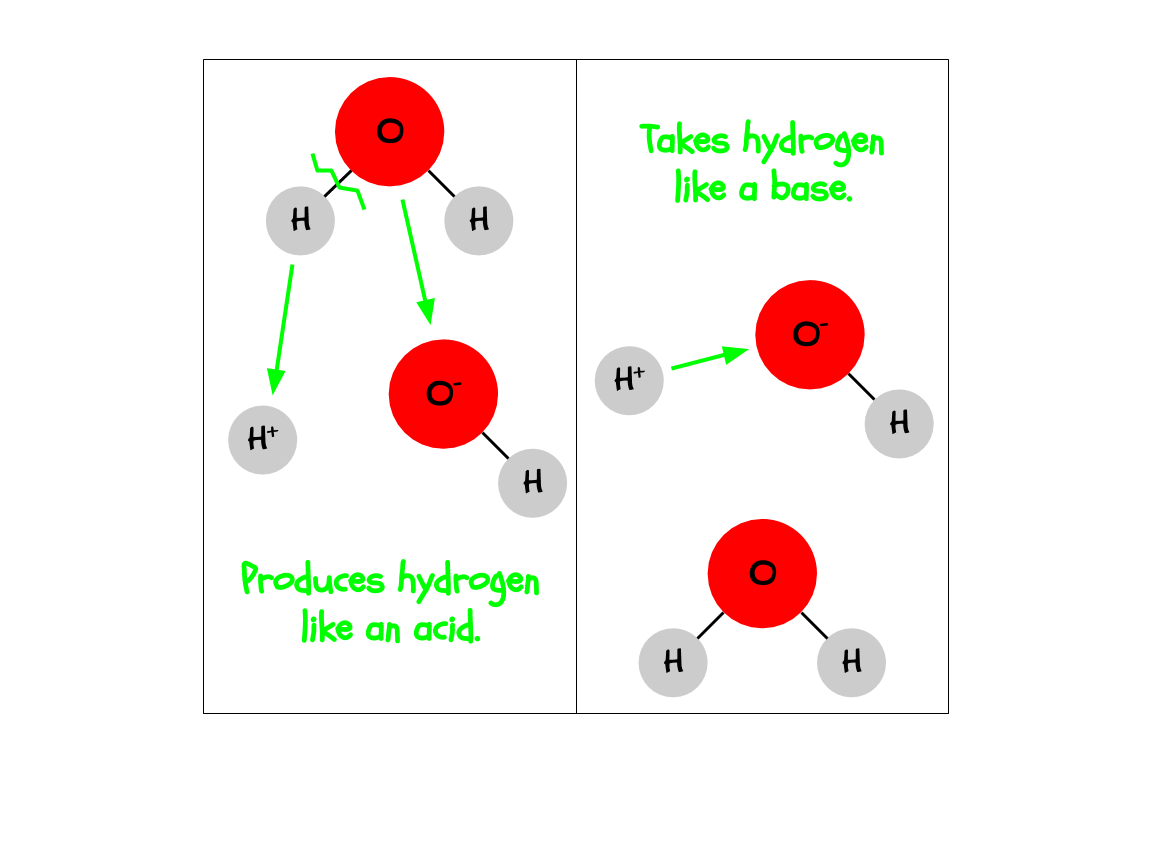
Chemically, an acid produces H+ ions in a solution, while a base takes H+ ions in a solution.
▲
An H+ ion is a positively charged atom of hydrogen. See the minipost The Smallest Particles for more information.
Measuring acidity/basicity
A measurement called pH determines how acidic or basic a substance is. pH is a scale from 0-14, with 0-6 being acidic, 7 being neutral, and 8-14 being basic (also called alkaline).
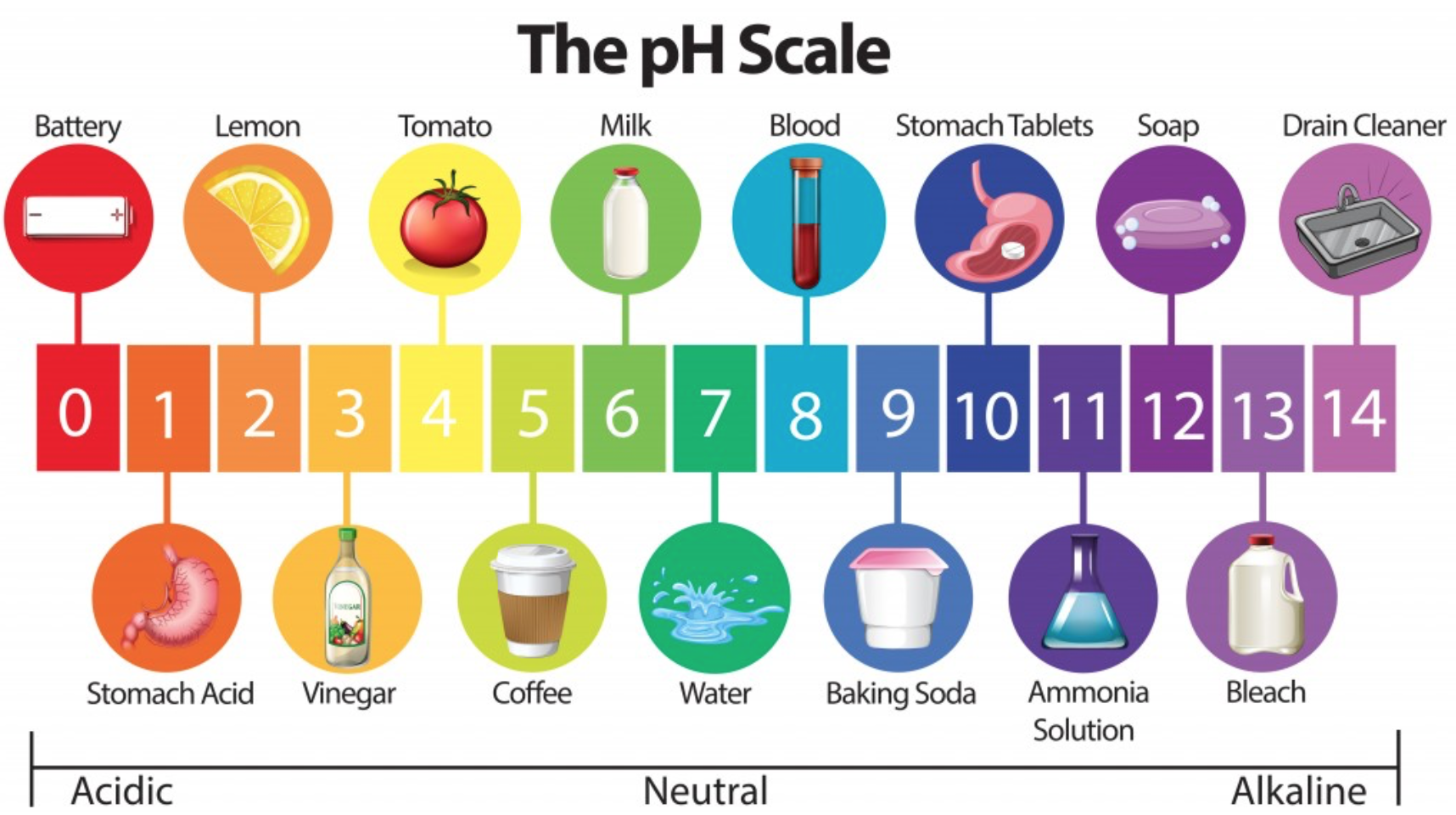
Image credit: scienceabc.com
Depending on who you ask, pH can stand for “potential hydrogen,” “per hydronium,” or “power of hydrogen.” Basically, the lower the number, the more hydrogen is produced, and the more acidic it is.
▲
See pH Math section below for a more complete description of what the number represents.
Certain substances are called pH indicators. One you can make at home is a cabbage juice indicator. Cabbages (and most purple-ish produce) have a pigment called anthocyanin in them that changes color with pH.
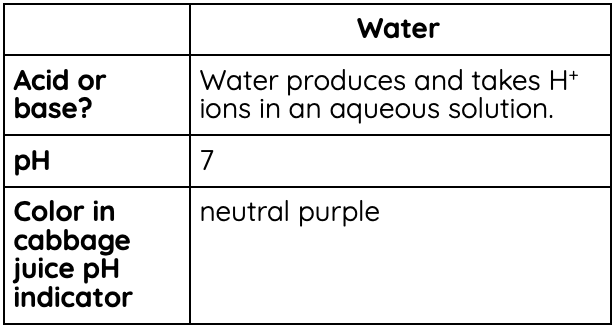
Is water acidic or basic?
Water, being the incredible anomaly that it is, is both an acid and a base. The H+ and OH- in H2O are constantly separating and coming back together, which means it is both producing H+ and taking H+. Thus, it fits both definitions.
Why pH matters
Acids and bases are everywhere. For example, we rely on stomach acid to digest our food and we use bases such as soap and ammonia to clean. pH levels are also very important when it comes to the environment.
Many ocean-dwelling creatures need their watery habitats to be at a certain pH level to survive. When burning fossil fuels releases CO2 into the atmosphere, carbonic acid (H2CO3) is formed. This acid can lower the pH of the ocean and harm marine organisms. Check out the Ocean Acidification Demo for more information and some hopeful solutions.
pH Math (for more advanced students)
The math for pH is a little more advanced (you probably need to be familiar with exponentials). pH is a logarithmic scale. A logarithm is the inverse of an exponential function.
Here’s one way to think about it: there is one (potential) H+ ion for every 10n molecules of the solution, where n is the pH value.
If there is a solution with a pH of 1, then for every 101 (10) molecules of solution, there is one hydrogen. That’s a lot of hydrogen, so the solution is very acidic.
On the flip side, if we have a solution with a pH of 14, you’ll only find a hydrogen ion every 1014 (100,000,000,000,000 or 100 trillion) molecules of solution. Hydrogen in this case is extremely hard to come by, so the solution is basic.