The Penny Polarity Experiment
2020-06-20
Learn why water sticks to itself by comparing it to other liquids.
Questions to explore:
- What is the difference between a polar and a nonpolar liquid?
- Why does water stick to itself?
- What is surface tension?
What you'll need:
- Two (2) pennies
- Two (2) eyedroppers
- Small cup of rubbing alcohol OR vegetable oil
- Small cup of water
- One (1) towel
What to do:
- Place both pennies face-up on the towel.
- Use the eyedropper to place drops of alcohol or oil, one at a time, on the face of one penny. Record how many drops you can place before the liquid overflows the edge of the penny.
- Repeat Step 2 with water.
- Compare your results. Which liquid were you able to fit more drops of on the penny?
How it works:
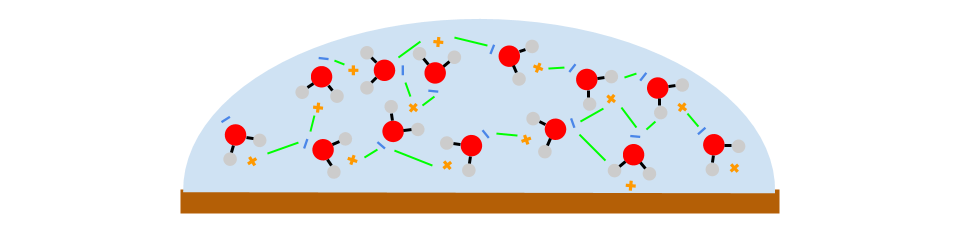
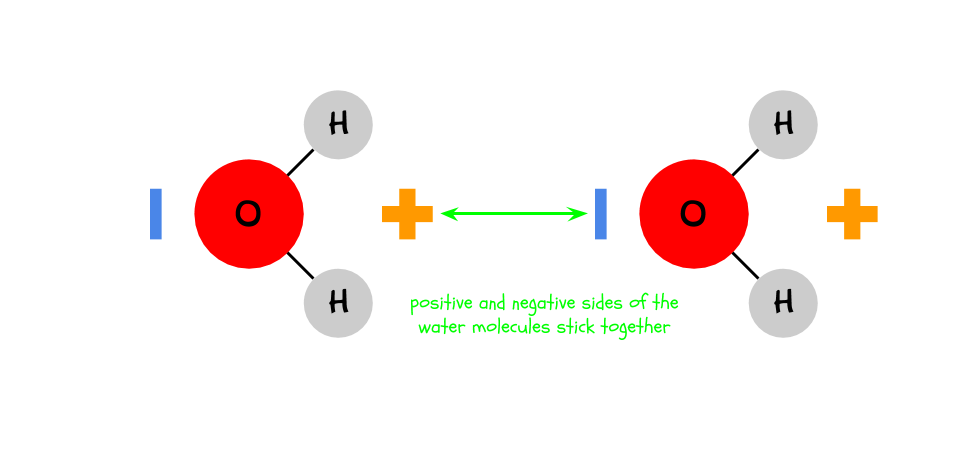
Water (H2O) is a polar molecule.
▲
See the polarity minipost for more details.
This means it has a positive and a negative end. Isopropyl alcohol and vegetable oils are relatively nonpolar molecules, meaning that they do not have a distinct positive or negative end.
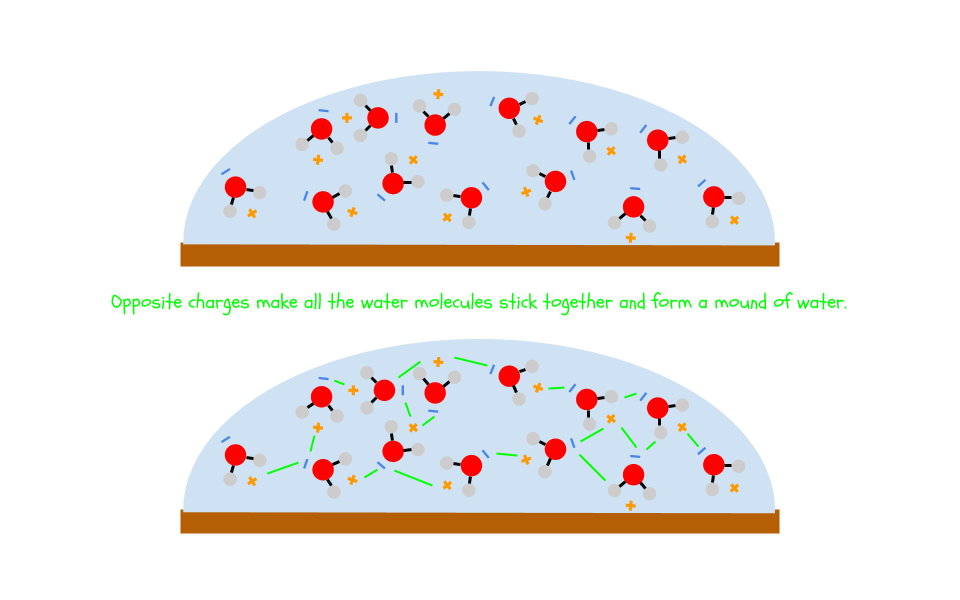
You probably noticed that the penny could hold more drops of water than drops of alcohol or oil. This is because the polarity of water causes it to stick to itself. Because opposite charges attract,
▲
Check out the Balloon & Stream Demo for another exploration of opposite charges.
the negative and positive sides of different water molecules stick together.
The water molecules on the outside of the bubble you see on the penny are so “sticky” that they can actually defy gravity and form a mound of water. This effect is called surface tension.
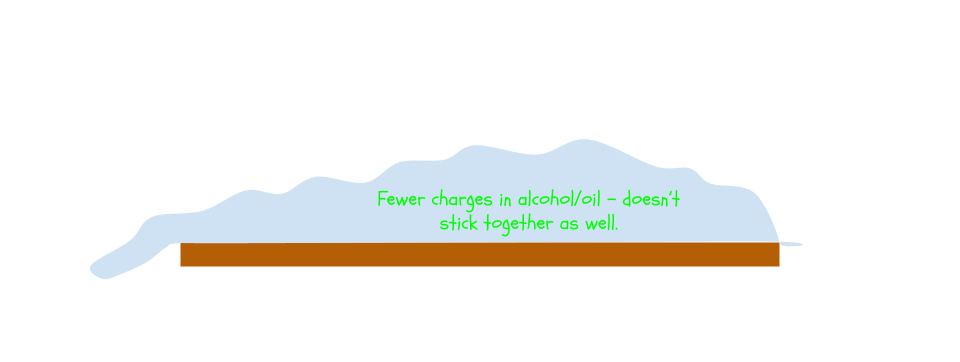
Alcohol and oil, being fairly neutrally-charged all around, cannot stick to themselves nearly as well as water can. Therefore, the “mound” of alcohol/oil succumbs to gravity and overflows much sooner than water.