Gummy Bear Osmosis
2020-06-20

Model water’s movement within our cells using gummy candy.
Questions to explore:
- How does water travel?
- Can water pass through seemingly-solid surfaces?
What you'll need:
- 2-4 gummy bears ▲ More if you want to eat some!
- 2-4 small bowls/containers
- Masking tape
- Marker
- Scale
- Measuring cup
- Tablespoon measure
- Spoon
- Tap water
- Salt
- Distilled water (optional)
- Corn syrup (optional)
What to do:
- Put 100 mL of tap water in each of two small bowls. Use the masking tape and marker to label one bowl “control” and the other “salt.”
- Add a tablespoon of salt to the bowl labelled “salt”. Mix until dissolved.
- If you have distilled water, label another bowl “distilled” and add 100 mL of distilled water.
- If you have corn syrup, label another bowl “syrup” and add 100 mL of corn syrup.
- Use the scale to weigh a gummy bear. Record its mass in grams.
- Place a gummy bear in each bowl. Try to fully submerge them.
- Wait at least 24 hours.
- Spoon the gummy bears out of the solutions and weigh them again. What do you notice? Did the masses change? What did different solutions do to the gummy bears?

How it works:
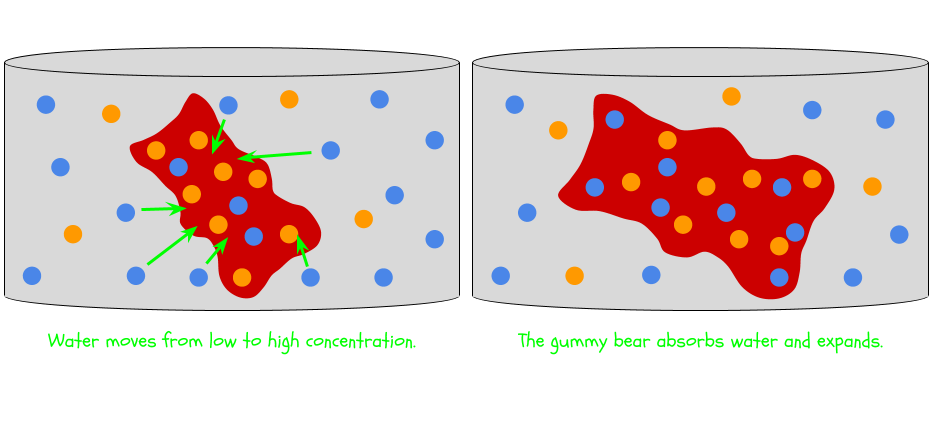
Osmosis is the movement of water across a membrane from an area of low concentration to an area of high concentration. Let’s break this definition down.
First of all, osmosis is the movement of water. The masses of the gummy bears change because water is moving in or out of them. The water is in the gummy bear itself, and in all the solutions (tap water, distilled water, salt water, and cornstarch).
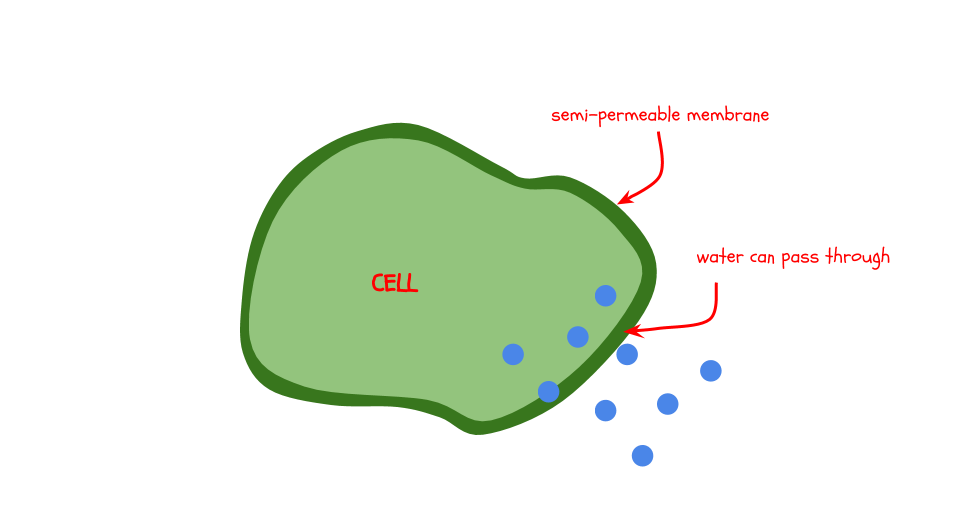
The membrane is what the water moves through. Membranes often seem solid, but they allow some things (but not all) to pass through them. This property is called being semi-permeable. The gelatin structure of a gummy bear is semi-permeable. The membranes that surround our cells are also semi-permeable. This experiment models how water moves in and out of our cells and keeps us alive.
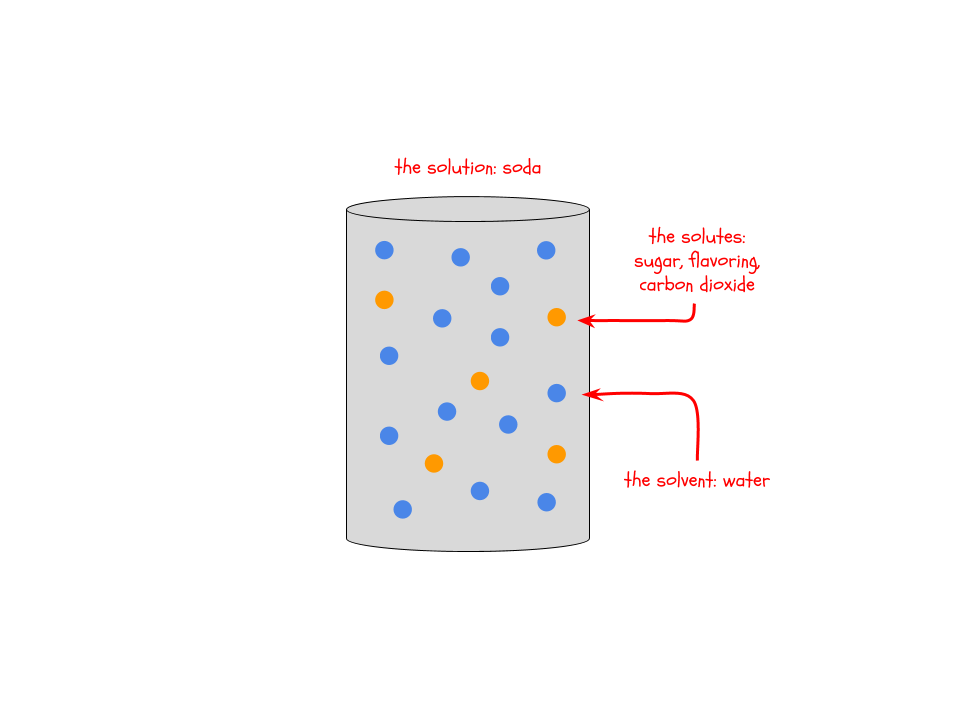
 Low or high concentration refers to the solutions on either side of the membrane (in the gummy bear and outside of the gummy bear). A solution is a liquid mixture in which one thing is dissolved in another thing. For example, soda is a solution of sugar, flavoring, and carbon dioxide (the bubbles) dissolved in water. The things that are dissolved (sugar, flavoring, carbon dioxide) are called solutes and the thing that they are dissolved in (water) is called the solvent
Low or high concentration refers to the solutions on either side of the membrane (in the gummy bear and outside of the gummy bear). A solution is a liquid mixture in which one thing is dissolved in another thing. For example, soda is a solution of sugar, flavoring, and carbon dioxide (the bubbles) dissolved in water. The things that are dissolved (sugar, flavoring, carbon dioxide) are called solutes and the thing that they are dissolved in (water) is called the solvent
 Gummy bears are technically solutions where a lot of sugar is dissolved in a little bit of water. Let’s look at the gummy bear in tap water. The solution inside the gummy bear has a higher concentration: there is a lot of solute (sugar) and not much solvent (water). But outside the gummy bear, the tap water solution has a very low concentration. It is mostly solvent (water) and a little bit of solute (minerals in the tap water).
Gummy bears are technically solutions where a lot of sugar is dissolved in a little bit of water. Let’s look at the gummy bear in tap water. The solution inside the gummy bear has a higher concentration: there is a lot of solute (sugar) and not much solvent (water). But outside the gummy bear, the tap water solution has a very low concentration. It is mostly solvent (water) and a little bit of solute (minerals in the tap water).
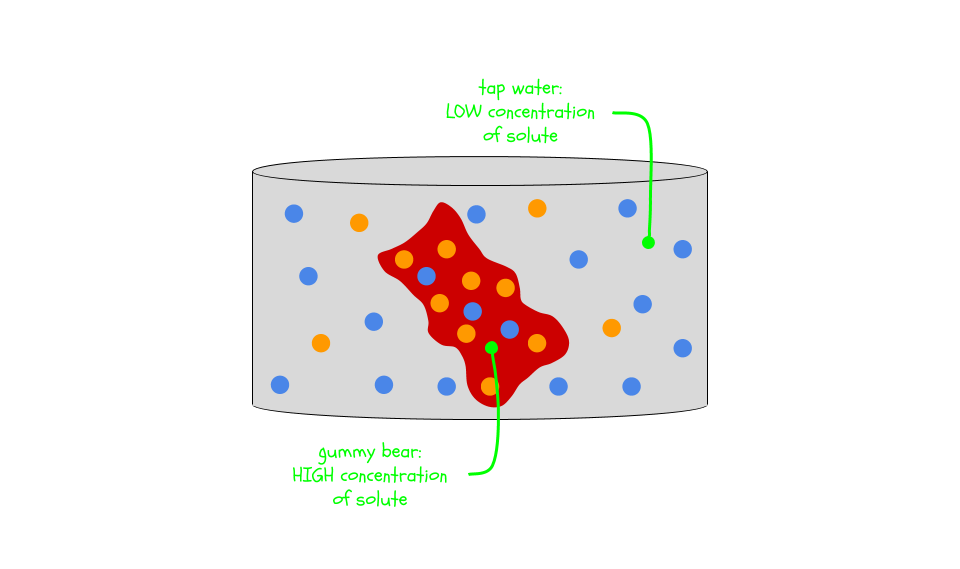 Water, the solvent in both cases, wants to even things out. So it moves from the area of low concentration (the tap water) to the area of high concentration (the gummy bear). You’ll probably see the gummy bear grow as a result. The gummy bear absorbs water and expands until the concentrations are more equal.
Water, the solvent in both cases, wants to even things out. So it moves from the area of low concentration (the tap water) to the area of high concentration (the gummy bear). You’ll probably see the gummy bear grow as a result. The gummy bear absorbs water and expands until the concentrations are more equal.
 If you used distilled water, the gummy bear probably grew even more. Distilled water doesn’t have a lot of the minerals that tap water has, so it is even lower concentration. Even more water would move into the gummy bear.
If you used distilled water, the gummy bear probably grew even more. Distilled water doesn’t have a lot of the minerals that tap water has, so it is even lower concentration. Even more water would move into the gummy bear.
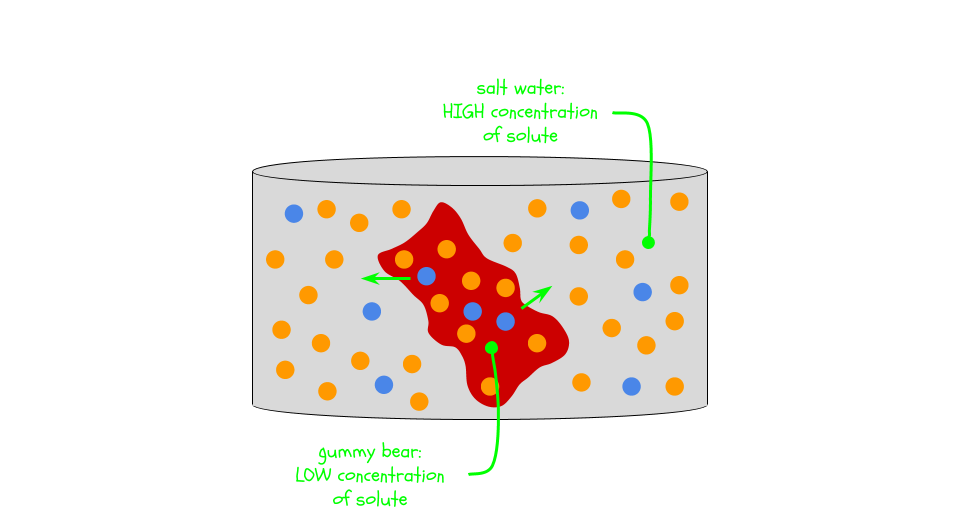
If you used corn syrup and salt water, the gummy bear probably didn’t grow as much or it might have shrunk. Corn syrup (sugar dissolved in water) and salt water (salt dissolved in water) are both very high concentration solutions. Water might have left the gummy bear (the lower concentration in this case) and caused it to shrink.